Bioequivalence Studies
Bioequivalence Studies
Bioequivalence studies determine whether generic drugs provide similar therapeutic effects as brand-name drugs. They compare bioavailability by measuring active ingredient concentration in the bloodstream to confirm similar absorption, distribution, metabolism, and elimination. Regulatory agencies like the FDA and EMA require these studies for generic drug approval. Factors such as formulation, patient variables, and drug interactions can influence bioequivalence. Thorough analysis ensures that patients receive the same benefits from generics as from the original drug.

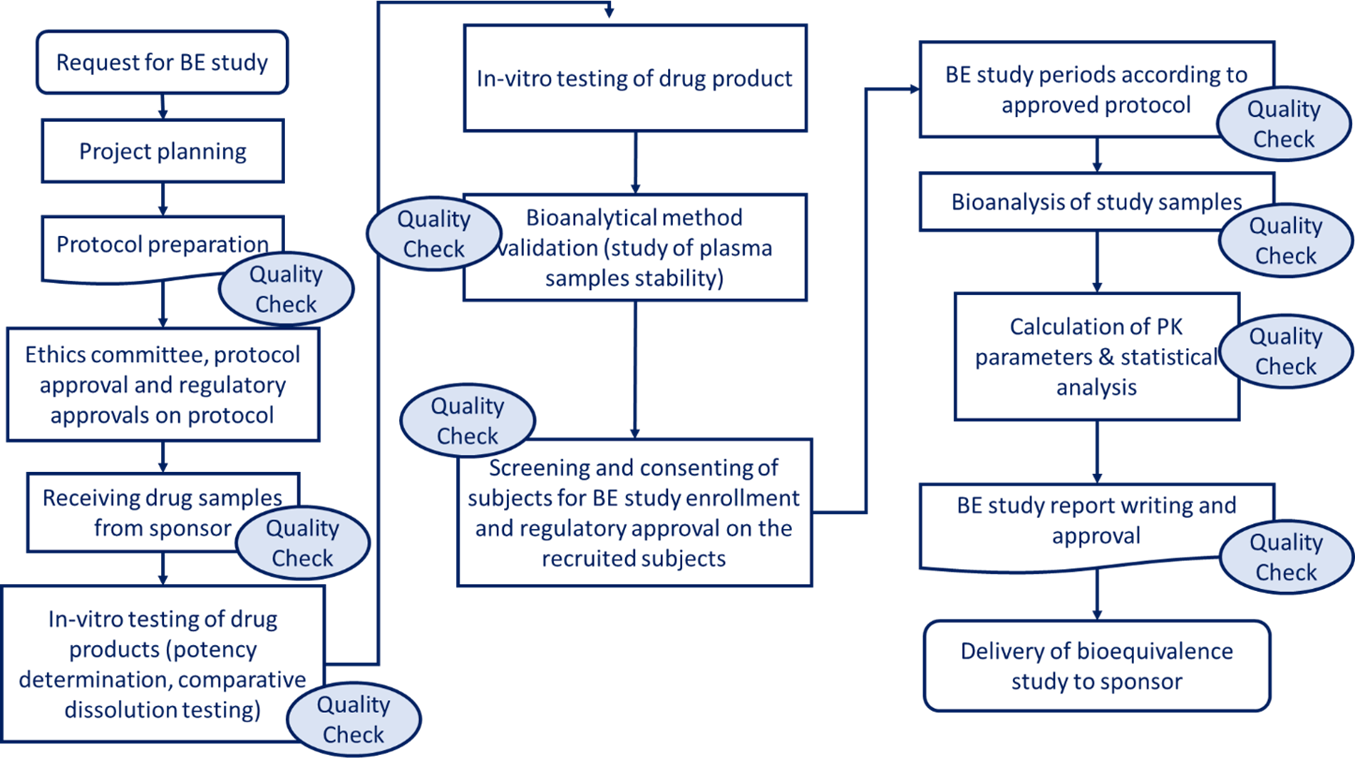
Project Scheme
GRC has conducted over 1,200 bioequivalence and bioavailability studies for generic drug registration. Their trained team supports study design through final reporting, adhering to GCP and GLP standards to comply with EDA, EMA, FDA, and ICH guidelines. GRC maintains ethical and scientific standards to ensure accurate, regulatory-compliant results for global generic drug approval.